- What is the difference between ECM-based and ECM-free systems in 2D and 3D cell culture?
- When are ECM-derived cues required for tissue organization and differentiation in 3D models?
- How do researchers choose between ECM-based and ECM-free culture formats for their experiments?
When setting up a 3D cell culture experiment, one of the first choices researchers face is whether to use an extracellular matrix (ECM) scaffold or an ECM-free format. ECM-based materials such as Matrigel and Geltrex have long been used to provide basement membrane signals that support adhesion, polarity, and tissue organization. At the same time, ECM-free ultra-low-attachment (ULA) approaches have gained traction as alternatives that enable spheroid and organoid formation driven primarily by cell-cell interactions rather than matrix-derived cues. The differences between these approaches can help researchers decide which system is most appropriate for their experimental goals:
- ECM-based systems (e.g., Matrigel, Geltrex, or similar) are typically used when differentiation, epithelial polarity, or tissue-level architecture is required. Applications such as mature organoid culture, blood-brain barrier (BBB) models, and stromal-tumor interaction studies often depend on ECM-derived signals.
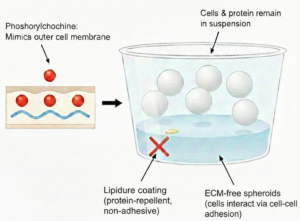
- ECM-free ultra-low-attachment (ULA) systems (e.g., Lipidure-coated plates) are often chosen for studies that call for uniform spheroid size, reduced biological variability, and compatibility with high-throughput workflows. These formats are well suited for compound screening, cytotoxicity assays, and applications where ECM-dependent phenotypes are not required.
In this article, we compare ECM-based and ECM-free culture approaches across common 2D and 3D workflows, including surface coatings, embedded cultures, dome-based workflows, and Transwell applications, alongside ECM-free ultra-low-attachment (ULA) systems such as Lipidure-coated plates. By highlighting the strengths and limitations of each approach, we aim to help researchers select the format best suited to their experimental goals.
Biological Considerations for ECM-Based and ECM-Free 3D Systems
A central biological consideration in 3D culture model design is how the presence or absence of an ECM scaffold influences cell behavior. Matrigel and its “cousin” Geltrex contain laminin, collagen IV, entactin and heparan sulfate proteoglycans, provide signals that support morphogenesis, epithelial polarity, and lineage specification. Organoids derived from iPSCs or patient tissues often rely on these ECM cues to assemble into structured microtissues. However, alternative strategies such as organoids-by-assembly or assembloids, in which pre-differentiated cell types are combined in controlled ratios, demonstrate that ECM scaffolds are not strictly required for organoid formation in all contexts.
On the other hand, ULA systems including Lipidure-coated plates are engineered to deliberately avoid ECM involvement. Lipidure is a synthetic polymer that was originally developed for medical and biocompatible surface applications. It is based on phosphorylcholine, which is the hydrophilic headgroup found in cell membranes. Lipidure’s structure therefore mimics the outer surface of living cells, creating a non-adhesive, protein-repellent surface that prevents cell attachment and matrix adsorption. In ULA cultures, cells interact primarily with each other, forming spheroids through cell-cell adhesion rather than ECM-mediated organization.
In our experience supporting a range of organoid and spheroid workflows, we have found that reproducibility is key when selecting 3D culture formats. The biological complexity of basement membrane extracts such as Matrigel and Geltrex can introduce lot-to-lot variability that affects organoid consistency across experiments. For this reason, ECM-free ULA approaches are often explored as a complementary strategy to reduce matrix-driven variability and support more standardized organoid production. Another advantage of ECM-free ULA systems is that they avoid the use of animal-derived materials, which can be important for workflows that require defined or animal-component-free culture conditions.
With these biological considerations in mind, we will now look at how ECM-based and ECM-free approaches are implemented across common 2D and 3D culture workflows.
2D Applications: Coating and Additives
Coating-based approaches represent the simplest use of ECM cues, which can be extended into fully 3D environments through embedded and dome-based culture formats. When used as a surface coating, Matrigel or Geltrex provides a biologically relevant substrate that improves attachment, phenotype stability, and assay robustness for primary cells, stem cell-derived cells, and certain sensitive immortalized lines. In such setups, the matrix forms a thin layer containing laminin-rich components that support adhesion and cell polarization. As alluded to in our previous article (see Similarities table), Matrigel and Geltrex are largely interchangeable for most applications.
In some workflows, a researcher might choose to supplement the coating with additional ECM proteins such as laminin, collagen IV, or fibronectin to mimic tissue-specific basement membrane compositions. These enriched coatings can help to stabilize long-term phenotypes or support specialized assays where native ECM signals are critical. This contrasts with ULA coatings, which are intentionally non-adhesive and therefore unsuitable for adherent 2D cultures, but are well suited for 2D suspension applications and can be applied to standard multiwell plate formats, including 6-, 12-, and 24-well plates.
Transwell Applications
Matrigel and Geltrex can also be applied to Transwell inserts to support barrier models and co-cultures. When used to coat the porous membrane in a Transwell apparatus, these materials help cells establish a stable, well-attached monolayer that is suitable for barrier and transport assays, including BBB models.
As described in our recent article about studying biological barrier function, Transwells are widely used for tumor-stromal interaction studies, immune cell migration assays, and permeability workflows. They may also be used in ECM-free systems, for example, spheroids generated in ULA plates can be transferred onto ECM-coated inserts for transport or drug-response studies.
Embedded 3D Cultures
In embedded 3D cultures, cells are mixed directly into cold Matrigel or Geltrex before gelation (setting), resulting in full encapsulation within a 3D ECM scaffold. This approach is often used by organoid researchers, in stem cell differentiation workflows, and in invasive tumor models. Since cells are fully surrounded by the matrix in this format, they experience local differences in ECM density and access to matrix-associated cues. These differences can influence how cells differentiate or organize within the gel, since cells positioned deeper in the matrix may experience different levels of ECM components than those closer to the surface.
Advantages of this approach include physiological relevance, support for multi-lineage differentiation, and compatibility with long-term studies. The limitations include the need for more complex imaging, slower diffusion of nutrients and compounds, and reduced suitability for high-throughput screening.
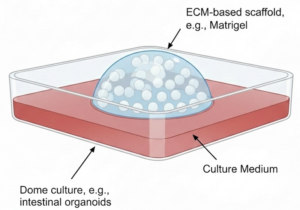
Dome Cultures
To create a dome culture, a small droplet of cells suspended in Matrigel or Geltrex is deposited onto a culture surface and allowed to solidify into a hemispherical structure before culture medium is carefully added without disturbing the dome. This format creates a localized 3D microenvironment that can support complex tissue organization, particularly for intestinal, neural, and tumor-derived organoids. However, dome-based workflows are highly sensitive to ECM concentration, temperature, and lot-to-lot variability, making them challenging to standardize across experiments and laboratories. Even small differences in dome volume or gelation can result in substantial variation in organoid size and morphology.
In contrast, ECM-free ultra-low-attachment systems promote highly uniform spheroid formation across wells, largely independent of matrix handling or lot-specific effects. This consistency makes ULA approaches particularly attractive for applications that require reproducibility, scalability, and quantitative comparisons.
Which Format Will You Choose? Let Tempo Support You!
Are you unsure about whether the ECM-based or the ECM-free system is best suited for your specific application? For guidance on selecting culture formats or establishing organoid and spheroid assays, please contact our Scientific Support Team; you can also submit images or experimental questions for our review (here).
—
Karen O’Hanlon Cohrt is an independent Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen relocated to Denmark where she held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Karen has been a full-time science writer since 2017, and has since then held numerous contract roles in science communication and editing spanning diverse topics including diagnostics, molecular biology, and gene therapy. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for learning helps her to keep abreast of exciting research developments as they unfold. Karen is currently based in Ireland, and you can follow her on Linkedin here.