Most of us were introduced to phagocytosis as a cellular event where dead host cells, microbial cells or their components, or other foreign bodies are engulfed and often destroyed by specialized cells known as phagocytes. During my undergraduate studies, phagocytosis was a small topic within immunology and macrophages were the crème de la crème of phagocytes, patrolling the body’s tissues for foreign invaders, much like security guards patrolling their territories.
The archetypal phagocyte resembles an amoeba, and a very simplified process of phagocytosis including destruction of the engulfed material is shown in Figure 1 below.
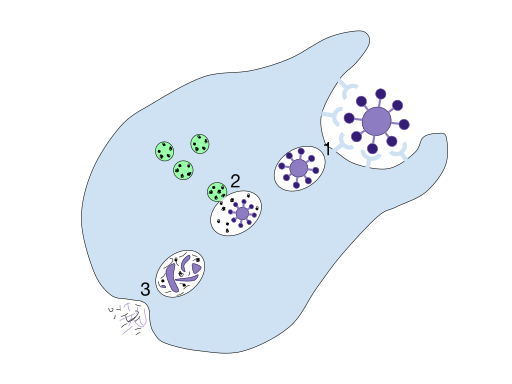
Figure 1: A simplified illustration of phagocytosis and destruction of the engulfed material. 1. A phagocyte recognizes certain antigens present on some particle/pathogen and ingests it, and a phagosome is formed. 2. The phagosome fuses with a lysosome to form a phagolysosome, and the particle is broken down by digestive enzymes stored in the lysosome. 3. The remains of the digested particle are expelled by the cell.
The Phagocyte – a Jack of Many Trades
While the processes of engulfment and phagosome formation are common to all phagocytes, what happens next may vary depending on the type of phagocyte in question. Moreover, phagocytes boast many roles that extend far beyond simply engulfing pathogens and dead cells, and phagocytes themselves are implicated in a number of important human diseases, such as: Chronic Granulomatous Disease and other immunodeficiencies, Alzheimer’s, Parkinson’s and a range of psychiatric conditions ranging from eating disorders to Schizophrenia.
So what do phagocytes do besides engulfing and sometimes destroying dead cells and pathogens? Let’s take a look at some of their many functions.
Initial Pathogen Encounter
Here, we refer primarily to dendritic cells (DC) and resident macrophages, which are among the first immune cells to interact with invading microbes. These cells are abundant in the interstitial fluid, where they continuously patrol for pathogens. Both cell types are capable of phagocytosing in the absence of a specific immune response, making them highly important rapid responders in the face of microbial threat.
Calling for Backup
Although necessary, the activity of resident macrophages alone is rarely sufficient to halt an infection, and the recruitment of other immune cells is imperative. Following phagocytosis, macrophages and DCs release a suite of cytokines and chemokines to stimulate and regulate the downstream immune response. Macrophages release TNF-α and interleukin 1 (IL-1) and the major class of DCs secretes IL-12. In addition to cytokines and chemokines, the release of H2O2 by the NADPH oxidase (known as the oxidative burst) by macrophages also serves as an important signal for downstream immune responses.
Pathogen Recognition
Phagocytes and other immune cells recognize pathogens by virtue of pathogen recognition receptors (PRRs) present on their surface. These receptors recognize corresponding pathogen-associated molecular patterns (PAMPs) on pathogens, which are typically components of bacterial (e.g., LPS) or fungal (e.g., β-glucan) cell walls. Although the recognition of pathogens via PRRs is much less precise than antibody-driven pathogen recognition, the interaction between a PRR and a PAMP and subsequent phagocytosis are sufficient to kick start an immune response that will be amplified further in parallel with a specific antibody-mediated or humoral response. PPR-mediated responses are general e.g., a general antibacterial response, as opposed to a species-specific response based on immunological memory e.g., an anti-TB response.
The toll-like receptors (TLRs) are by far the most important of the PRRs, and these collectively recognize a wide range of PAMPS, often working as dimers, with some TLRs even working in concert, to activate specific signaling cascades and gene expression programs that eventually lead to effective immune responses against the offending pathogen. Other important cell surface PRRs expressed by phagocytes include the C-type lectin receptors e.g., the mannose receptor expressed by macrophages and immature DCs. In additional to cell surface-bound PRRs, a number of cytoplasmic and plasma-borne PRRs exist, such as the NOD-like receptors and mannose binding lectin, respectively.
Pathogen Killing – Inside and Out
One of the most critical functions of phagocytes is killing of pathogens. Intracellular killing involves the prior ingestion of microbes followed by killing inside the phagocyte. The most effective means of intracellular killing occurs as an oxygen-dependent process, whereby intracellular oxygen consumption increases upon ingestion, leading eventually to the release of toxic reactive oxygen species (the oxidative burst as mentioned above), which is mediated by the NADPH oxidase complex. The reactive oxygen species released to kill the pathogen are retained in specialized cellular compartments to avoid toxicity to the host. Intracellular killing may also occur independently of oxygen. Here the cell using a combination of lytic and proteolytic enzymes and chelating proteins e.g., lactoferrin to destroy the engulfed microbe.
Extracellular killing
occurs when phagocytes kill microbes outside of the cell. This occurs in macrophages that have been stimulated by interferon gamma to produce nitric acid. T cells, B cells, or other phagocytes may be the origin of the interferon gamma as part of an active immune response. Nitric acid is highly toxic and kills cells in the nearby vicinity.
Maintaining Cellular Homeostasis
Although most research efforts concerning phagocytes seem to focus on pathogen removal and immunity, we shouldn’t forget that phagocytes are also extremely important in the maintenance of healthy tissues. They do this by engulfing and removing dead cells and damaged cells that result from tissue injury. Phagocytes are also responsible for clearing dying cells that display certain cell surface molecules during the final stages of apoptosis. Phagocytosis of such cells occurs without the elicitation of an immune response.
The Many Faces of Phagocytes
As alluded to above, phagocytes may be activated by different triggers in response to different threats or stimuli, and may act differently depending on their role(s) and location in the body. We recently compared two types of phagocytes; macrophages and microglia, but these two merely represent the tip of the phagocyte iceberg. Phagocytes can be placed into three categories: the professional, the nonprofessional and the specialized. In this post, we will take a closer look at the professional phagocytes to see what they do and don’t have in common.
The Professional Phagocytes
Professional phagocytes are named so because they possess specific receptors on their surfaces that allow them to recognize pathogens. All of the currently known professional phagocytes are some kind of white blood cell and include: macrophages, dendritic cells, neutrophils, monocytes, and mast cells. These cells share a common origin from stem cells in the bone marrow as depicted in Figure 2.
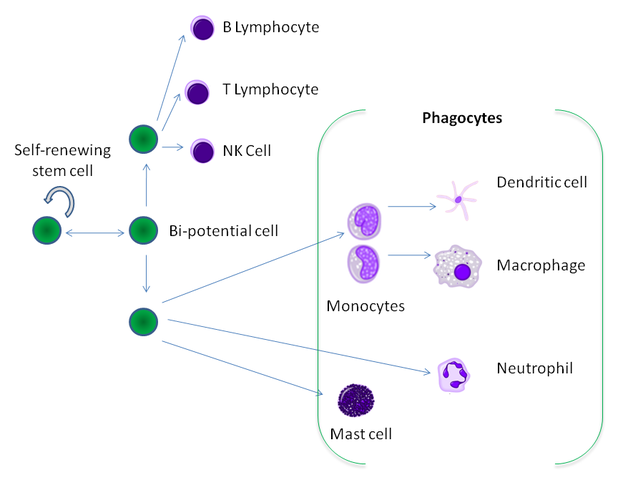
Figure 2: The hematopoietic origin of phagocytes from stem cells in the bone marrow.
All professional phagocytes, and indeed all phagocytes, share a few important features:
- The need and ability to distinguish healthy host cells from dying or damaged host cells and microbial cells
- The ability to ingest and digest (if relevant to the cell type) the foreign or pathogenic material
- The means to cope with an increase in biomass upon engulfing other cells or cellular components
- The need to protect itself and other host cells from toxic products used during microbial killing e.g., reactive oxygen species
But how do they differ? Let’s address this by taking a look at their functions in more detail.
Macrophages and DCs
While the initial act of engulfment and phagosome formation is similar for macrophages and DC, the consequences are distinct. Macrophages phagocytose with the sole purpose of destroying their engulfed targets, thus playing a critical role in clearance during the innate immune response to infection before a specific immune response sets in. DCs, on the other hand, collect antigens from their engulfed targets, which they transport and present to other specialized white blood cells e.g., lymphocytes in the lymph, mucosa-associated lymphoid tissues, or the spleen. Although macrophages, upon stimulation by interferon gamma, are also capable of presenting phagocytosed pathogen fragments to the T helper cells, DCs are arguably the most versatile of the professional antigen-presenting cells, and are essential for the activation of naive T cells in the lymph nodes.
Neutrophils
Neutrophils are one of the first cell types to be recruited during an infection (through the activity of H2O2, IL-8 and other signals), and are often referred to as the first line of response. Neutrophils are ordinarily present in large numbers in the blood vessels, but migrate to the site of infection upon perceiving the right signal. Migration of neutrophils out of the blood vessels is facilitated by the expression of adhesion molecules on epithelial cells, a process that is driven by TNF-α and IL-1 released from macrophages. Chemokines released by macrophages and DCs are also important in the transit of neutrophils between adjacent endothelial cells (diapedesis) and towards the site of infection (chemotaxis). Neutrophils are well known for their intracellular granules that contain an arsenal of proteolytic and bactericidal products. Neutrophils are short-lived, playing a critical role in the acute response to an infection, and once their mission is complete, they turn into pus cells and die.
Monocytes
Monocytes mature in the blood, and act both as phagocytes and antigen-presenting cells. Monocytes often circulate the blood but the majority remain in other tissues. When monocytes leave the bloodstream for other tissues and organs, they undergo a transformation to macrophages or DCs, depending on the stimulatory signal they receive. In contrast to neutrophils, monocytes that transform into macrophages at infection sites are long-lived, contributing to the response towards chronic infections.
Mast Cells
Mast cells, the only professional phagocyte not already mentioned here, possess toll-like receptors and interact with many other immune cells including DCs, and B and T cells, and secrete pro-inflammatory cytokines in order to support the implementation of an antibody-mediated immune response. Mast cells participate directly in the killing of gram-negative bacteria and are also capable of antigen presentation, although little is known about their role in this process.
The Story Continues
As you have probably gathered by now, phagocytosis is a broader topic than what many of us encountered during our undergraduate years. With many different types of phagocytes, seemingly distinct yet somewhat overlapping functions, and continuous new insights from research, it’s not a straightforward task to simply describe their similarities and differences. We hope this post gives you some insight into this fascinating world, and that you will stay tuned for future articles about the many faces of phagocytes!
Image Credits
- Figure 1. Mango Slices (own work). Sourced from Wikimedia Commons under the Creative Commons Attribution-Share Alike 4.0 International license.
- Figure 2. Graham Colm at English Wikipedia. Sourced from Wikimedia Commons under the Creative Commons Attribution-Share Alike 3.0 Unported license.
Article by Karen O’Hanlon Cohrt PhD. Contact Karen at karen@tempobioscience.com.
Karen O’Hanlon Cohrt is a Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen moved to Denmark and held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for writing helps her to keep abreast of exciting research developments as they unfold. Follow Karen on Twitter @KarenOHCohrt. Karen has been a science writer since 2014; you can find her other work on her portfolio.
