It’s a fibroblast-like cell adorned with long cytoplasmic processes that wrap around the endothelial cells in blood vessels, it controls blood flow through the blood vessels, and it is essential for normal brain function and development. Which cell type is it? Well, it’s a pericyte of course! Welcome back to our Cell of the Month series. This time, we focus on the pericytes, summarizing what they are, what they look like, and what they do.
So, what is a pericyte again?
Pericytes are multi-functional cells that wrap around the endothelial walls of the arterioles, capillaries, and venules. They are found in every vascularized tissue in the human body, and are estimated to cover between 22% to 99% of the endothelial cell surface. Pericytes are surrounded by the same basement membrane as endothelial cells, which they interact extensively with via “peg and socket” contacts through holes in the basement membrane or via paracrine signaling.
Pericytes were first identified about 150 years ago by French scientist Charles-Marie Benjamin Rouget who was studying the anatomical structure of capillaries in amphibians; he described them as perivascular cells that share the basement membrane with adjacent capillaries. Along with vascular smooth muscle cells, pericytes belong to the category of mural cells, i.e., cells that surround the endothelium of blood and lymphatic vessels and support their function. For many years, their biological role remained elusive for a number of reasons, including difficulties in studying pericytes in vivo, as well as challenges in isolating pure primary pericytes and the lack of a pericyte-specific marker.
Morphologically, pericytes have a distinct nucleus compared to the flat nucleus of endothelial cells. They harbor large mitochondria and contain long cytoplasmic processes that wrap around the capillary wall to regulate blood flow. They are highly contractible cells, owing to a unique cytoskeleton organization of actin filaments that allows active remodeling of their distal processes, which helps to ensure sufficient coverage of the endothelium as required.
Pericytes have diverse developmental origins
As a highly heterogeneous cell type, pericyte morphology and protein expression profiles vary greatly depending on their developmental origin and anatomical location. Pericytes in the brain, thymus, lungs, heart, liver, and gastrointestinal tract originate from the ectoderm, while pericytes in most other organs originate from the mesoderm.
Pericyte heterogeneity has hampered efforts to identify a specific single marker for their identification, but neural/glial antigen 2 (NG2) and platelet-derived growth factor receptor b (PDGFRβ) are widely used in combination with morphological- and counter-staining approaches to discriminate between pericytes and other cells.
While no universal pericyte marker yet exists, advances in single-cell analysis tools in recent tools have provided new insights into pericyte heterogeneity, and the repertoire of tissue-specific pericyte markers is growing (1).
A jack-of-all-trade cell type
Pericytes functions can roughly be grouped into four major categories, and their distribution and density among organs and vascular beds vary to meet tissue-specific demands. Although not discussed in detail here, pericytes have been shown to promote the expression of a variety of pro- and anti-inflammatory molecules that are secreted in response to pro-inflammatory stimuli, and recent research points towards a broad role for pericytes in modulating immune responses (2).
CNS homeostasis and blood-brain barrier regulation
The microvasculature of the central nervous system (CNS) shows the greatest pericyte-to-endothelial cell abundance. Here, pericytes form part of the neurovascular unit (NVU), a collection of cells that control the interactions between nerves and the brain vasculature to meet the brain’s energy requirements. The NVU is a fascinating aspect of current BBB research focusing on how to more accurately translate the human BBB for therapeutic development. You can read more about that in our previous article about in vitro BBB models for drug development here.
One essential role for pericytes in the brain is to control the passage of fluid and substances across the blood-brain barrier (BBB) into the parenchymal space. This is mediated by their physical interactions with endothelial cells that leads to the formation of a continuous chain-like barrier along the capillaries of the brain vasculature, as well as extracellular matrix deposition by pericytes (other cells within the NVU also play a role here), which helps to maintain the integrity of the vascular barrier. These critical functions are underscored by the fact that pericyte dysfunction and defective pericyte recruitment within the CNS, caused by aberrant paracrine signaling, e.g., as a result of disruptions to the PDGF-BB/PDGFRβ signaling pathway, lead to increased BBB permeability and reduced BBB function.
Not surprisingly given their vast abundance in the brain vasculature, pericyte-related vascular defects have been implicated in CNS diseases associated with BBB degradation, including Alzheimer’s, Parkinson’s, dementia, stroke, and others (3). Additional phenotypes that link pericyte dysfunction to CNS disease include neuron death and impaired coupling between neuronal activity and blood flow.
Other roles for pericytes within the CNS include blood vessel formation (angiogenesis), maintenance of adequate calcium levels, regulation of immune cell entry to the brain and control of brain blood flow.
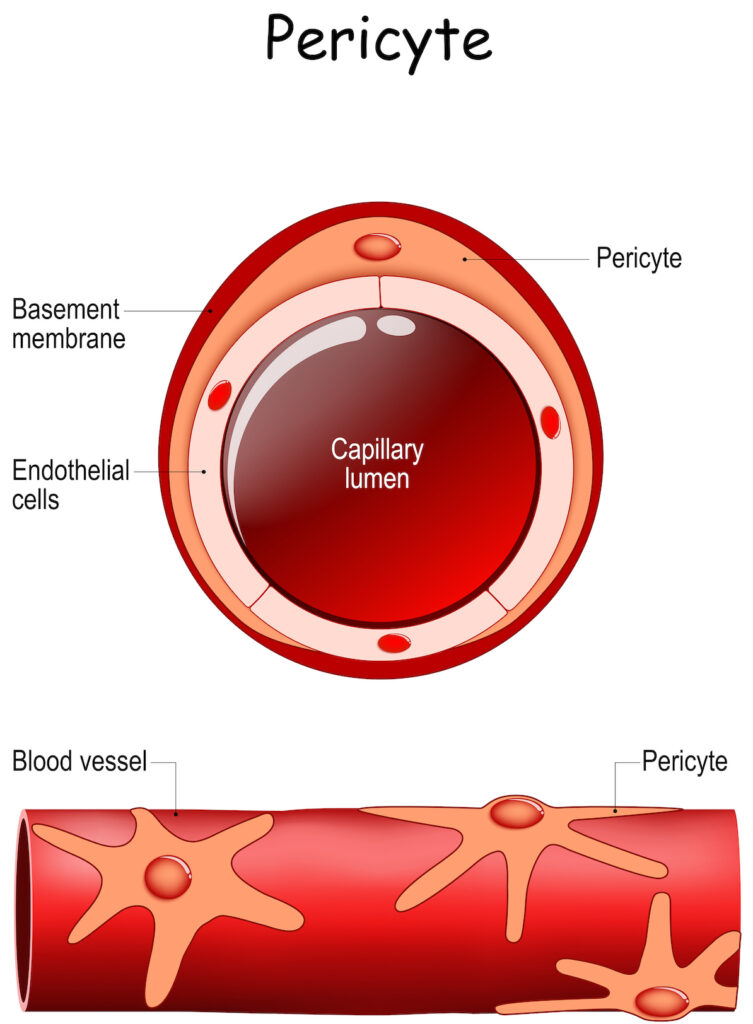
An illustration of Pericytes wrapping around a blood vessel and endothelial cells. To learn more about iPSC-derived Pericytes, visit Tempo-iPeri.
Angiogenesis and blood vessel stabilization
Research findings strongly suggest that pericytes play a crucial role in blood vessel growth and maturation, and that this role occurs via activation of transforming growth factor-β (TGF-β) signaling and production of the endothelial cell survival factor angiopoietin 1 (Ang1). In addition, the presence of pericytes within the endothelial tubules is considered to impart physical stability and support for endothelial tubule function in the earlier stages of angiogenesis.
Historically, it was believed that pericytes contributed primarily to the later stages of blood vessel formation. However more recent findings using the mouse retina as a model of angiogenesis have challenged that view. For instance, some studies have shown that pericyte growth occurs before the expansion of endothelial cells in the early stages of developmental angiogenesis, and that while angiogenic processes may be initiated within endothelial cells in the absence of pericytes, those cells fail to proliferate. Those findings support a model whereby pericytes are necessary for endothelial cell expansion. Other studies have shown that reduced pericyte coverage actually leads to enhanced endothelial cells proliferation.
Whether these discrepancies can be explained by differences in the experimental approaches used is unknown, but it becoming clear that the role of pericytes in angiogenesis is much more complex and dynamic than previously thought, and it is possible that their contribution to angiogenesis is tissue-dependant and dictated by their relative abundance to endothelial cells. Autoregulatory feedback loops in the pathways that regulate pericytes and pericyte-endothelial cell crosstalk, e.g., angiopoietin, PDGFRβ, and TGF-β may also explain some of the mysteries about the role of pericytes in angiogenesis.
Tissue repair and regeneration
Within the last 10-15 years, a role for pericytes in tissue repair and regeneration following injury has become apparent, following the discovery that pericytes possess stem-cell like properties.
Some researchers have described pericytes as being a precursor to mesenchymal stem cells (you can read more about mesenchymal stem cells in a previous article here), while others claim that pericytes and mesenchymal stem cells are one and the same. This is a hotly-debated topic and further scientific investigations are crucial to probe into the relationship between these two apparent cell types.
Despite the open questions regarding their stemness, the demonstrated stem-like properties and multi-functionality of pericytes has sparked huge interest in their therapeutic potential for tissue repair, particularly in the brain where they are most abundant. The fact that pericytes occupy the interface between the microvasculature and the brain puts them in the ideal location to promote repair and regeneration in response to various stress factors; they can act as a paracrine signaling hub in which they receive signals from within the brain tissue itself or from circulating blood.
In addition, pericytes residing on blood vessels in the brain can promote angiogenesis and drive inflammatory responses, trigger the influx of leukocytes to the site of injury, as well as phagocytose cellular debris following injury. The timed coordination of these responses can aid in the repair and regeneration of injured or diseased tissue, and research in animal models of disease suggests that this can occur in the brain (4). Further studies that include multi-cell type 3D models, for example organoids (which you can read about in our previous article here), should provide clearer insights on the exact contribution of pericytes to wound healing, and how this can be harnessed therapeutically.
Blood flow regulation
Controlling blood flow was one of the earliest documented roles for pericytes, which was hinted by their location on the outer capillary walls, their long cytoplasmic processes and their close contact with adjacent capillary endothelial cells.
Local modulation of cerebral blood flow, which is critical for normal brain function, occurs through a tightly-controlled process known as neurovascular coupling. Here, it is established vascular cells that possess contractile properties, such as smooth muscle cells and pericytes, bring about alterations in the vascular diameter, but exactly which cell type is responsible for initiating neurovascular coupling and where along the arterial–venous axis the coupling occurs remains unknown.
Emerging evidence suggests that cerebral blood flow is regulated at a capillary level, which further implicates pericytes, with much research ongoing in this area. One recent study carried out at Zilkha Neurogenetic Institute in Los Angeles addressed the contribution of pericytes in maintaining controlling cerebral flow using pericyte-deficient mice that had an approximately 30% reduction in overall pericyte numbers compared to wild-type mice. These mice had no apparent alterations in smooth muscle cells, measured blood flow responses, brain oxygenation, or neuronal metabolism and activity in the living mouse brain. A set of experiments that included real-time microscopy, brain oxygenation measurements, and in vivo microdialysis revealed that loss of brain pericytes in those mice coincided with dysregulated blood flow, which led to reduced brain oxygenation and neuronal metabolic stress (5).
Stay tuned!
While our understanding of pericytes has grown tremendously since their discovery some 150 years ago, many gaps remain in our understanding, e.g., their tissue-specific phenotypes and functions, temporal regulation, and response to microenvironments. Extensive future characterization of tissue-specific pericyte roles in human-relevant diseased vs. healthy states is also needed to fully delineate how they contribute to disease initiation and progression, and reveal how they may be harnessed therapeutically.
That was it for pericytes this time. Stay tuned for future articles where we will look at the emerging roles for pericytes in disease as well as our next Cell of the Month installment about brain microvascular endothelial cells!
References
- Baek SH, Maiorino E, Kim H, Glass K, Raby BA, Yuan K. Single Cell Transcriptomic Analysis Reveals Organ Specific Pericyte Markers and Identities. Front Cardiovasc Med. 2022 Jun 1;9:876591.
- Dabravolski SA, Andreeva ER, Eremin II, Markin AM, Nadelyaeva II, Orekhov AN, Melnichenko AA. The Role of Pericytes in Regulation of Innate and Adaptive Immunity. Biomedicines. 2023 Feb 17;11(2):600.
- van Splunder H, Villacampa P, Martínez-Romero A, Graupera M. Pericytes in the disease spotlight. Trends Cell Biol. 2023 Jul 18:S0962-8924(23)00111-3.
- Tachibana M, Yamazaki Y, Liu CC, Bu G, Kanekiyo T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-β pathology in amyloid model mice. Exp Neurol. 2018 Feb;300:13-21.
- Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017 Jul;18(7):419-434
Karen O’Hanlon Cohrt is an independent Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen relocated to Denmark where she held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Karen has been a full-time science writer since 2017, and has since then held numerous contract roles in science communication and editing spanning diverse topics including diagnostics, molecular biology, and gene therapy. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for learning helps her to keep abreast of exciting research developments as they unfold. Karen is currently based in Ireland, and you can follow her on Linkedin here.
