- How do Transwell assays help model biological barriers in the lab?
- What does TEER tell us about barrier integrity and tight-junction strength?
- How can these assays advance our understanding of drug transport and disease?
Biological barriers are critical for maintaining cellular homeostasis and protecting organs from harm caused by pathogens, drugs, and toxins. Like a country’s border crossing, a biological barrier decides who, or more so, what, gets through, and when. These specialized cellular interfaces, such as those in the brain, skin, kidney, and retina, regulate how nutrients, metabolites, and signaling molecules are exchanged between different body parts.
While we often think of barriers as static and unchanging, biological barriers are dynamic and responsive, and continually adapt to physiological cues and environmental stresses. Good methods for modeling barrier function in vitro are critical for understanding how drugs, chemicals, and environmental exposures affect barrier integrity and transport. Such models allow researchers to assess drug permeability, safety, and toxicity early in the discovery process, and also help reveal the cellular mechanisms that maintain or disrupt barrier function in health and disease.
The most common approach to studying barrier function is the Transwell assay. We previously described how this system is used to model the BBB. In this article, we will look at how the Transwell assay is used more broadly to model various barrier-forming cell types including brain microvascular endothelial cells (BMECs), which are the principal cell type forming the BBB, keratinocytes, retinal pigment epithelial cells (RPEs), and others.
So, what is a Transwell assay and how does it work?
In short, the Transwell assay is a widely used in vitro model of barrier formation, transport, and permeability. Here, epithelial or endothelial cells are typically cultured as a confluent monolayer on a porous membrane that separates two chambers: an upper (apical) and a lower (basolateral) compartment. As depicted for a BMEC monolayer in Figure 1, this setup allows controlled measurement of how solutes, nutrients, or drugs move across the barrier. The pore size of the membrane is critical and typically ranges from 0.3 to 1.0 µm; this is small enough to support cell attachment and tight-junction formation, but large enough to allow diffusion of signaling molecules or small solutes.
In both barrier and migration assays, extracellular matrix (ECM) components such as collagen, fibronectin, or laminin are often used to coat the porous membrane. These enhance cell attachment and influence differentiation, polarity, and junctional organization. Unlike cell migration or invasion assays, which use larger pores (typically in the 3 to 10µm range) to allow cell movement through the membrane, barrier assays focus on forming a continuous, impermeable cell layer to study transport across, rather than between, cells (1).
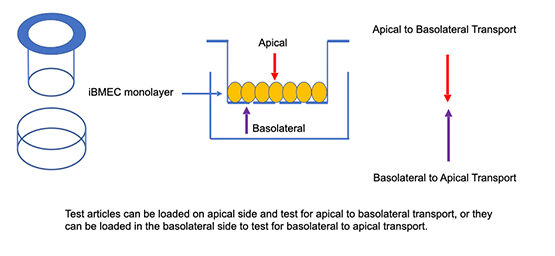
Figure 1: Schematic representation of the classical Transwell assay. The apical and basolateral chambers, on either side of a monolayer of human iPSC derived brain microvascular endothelial cells (iBMECs) that are cultured on a porous membrane, are shown.
While the Transwell has been widely used to model the BBB since the 1980s it has also been adapted for many other tissue types. Its cost-effectiveness, reproducibility, and compatibility with co-culture systems makes it a versatile platform for studying transport, permeability, and barrier integrity across diverse cell types.
What is a TEER assay and how is it used to measure barrier integrity?
The Transepithelial/Transendothelial Electrical Resistance or TEER assay is a quantitative method often used alongside Transwell systems to measure barrier integrity. TEER measures electrical resistance across a cell monolayer, providing a non-invasive, real-time readout of tight junction formation and barrier strength. To run a TEER assay, electrodes are placed in upper and lower chambers of a Transwell apparatus (Figure 1). The chambers are then filled with a conductive media, which allows the electrodes to detect ion flow through the cell layer and membrane. Generally speaking, higher TEER values indicate stronger, more selective, resistant barriers, while lower values reflect several possible scenarios including incomplete junctions or compromised integrity, or compromised monolayer tight-junction (cell-to-cell adhesion) functions, or alternatively that the barrier contains pores.
Because TEER measurements can be repeated without disturbing the cells, this approach is ideal for tracking barrier maturation and monitoring how drugs, inflammatory signals, or environmental stressors impact permeability over time. TEER is now also integrated into dynamic organ-on-chip platforms that replicate physiological flow and shear stress. However, TEER measurements can vary considerably between studies due to differences in electrode configuration, cell type, and experimental conditions. Reported values can range widely, from several hundred to over one thousand ohm/cm², even for similar barrier models. To improve comparability, TEER readings are typically normalized to surface area and should be interpreted in the context of species, cell origin, and buffer composition.
What cell types are often studied using Transwell and TEER assays?
Transwell and TEER assays are applied across a wide range of epithelial and endothelial cell types to study organ-specific barriers and transport processes. Below are some of the most common barrier-forming cell types examined using these assays with selected recent examples from the literature:
Brain Microvascular Endothelial Cells (BMECs): Transwell assays remain an important in vitro model for studying the BBB. In these systems, BMECs are often co-cultured with astrocytes and pericytes, which are the cell types that provide essential biochemical cues and support tight-junction formation. Such co-cultures better reproduce the selective permeability and low paracellular transport characteristic of the BBB, making them valuable for assessing drug delivery, neuroinflammation, and barrier dysfunction.
While conventional Transwell setups are static, newer microfluidic BBB-on-a-chip platforms now integrate these same cell types under dynamic flow, combining physiological shear stress with co-culture complexity to yield properties that require mechanical flow. For example, researchers in Spain and Chile recently developed a BBB-on-a-chip incorporating human endothelial cells, astrocytes, and pericytes within a 3D extracellular matrix and integrated micro-TEER electrodes for real-time barrier monitoring. The authors reported high TEER values indicative of robust barrier integrity, comparable to those seen in established BBB model systems (2). While BBB-on-a-chip models represent an important advance in studying BBB integrity and transport, reproducibility and validation remain ongoing challenges as researchers continue to refine their design and application.
Keratinocytes: Keratinocyte-based Transwell models, designed to mimic the human skin (epidermal) barrier, are frequently used to assess irritation caused by drugs or cosmetics, as well as wound healing, and topical or transdermal drug delivery. In these setups, human keratinocytes are typically cultured on permeable membranes at an air-liquid interface. Validated reconstructed human epidermis (RHE) models, which are functionally analogous to the Transwell insert format, include EpiDerm™, EpiSkin™, and others. These standardized keratinocyte-based systems are used in regulatory testing under OECD Test Guideline 439 for skin irritation and OECD TG 431 for skin corrosion (3, 4).
Kidney epithelial cells: Kidney epithelial cell lines, such as MDCK and HK-2, are commonly cultured on Transwell membranes to model tubular transport, reabsorption, and epithelial polarity. These assays are useful for studying nephrotoxicity and kidney physiology by allowing directional transport measurements between the upper and lower chambers.
Recently, a Transwell-based model using human tubuloid-derived epithelial cells was developed to represent distal nephron segments. The resulting epithelia formed polarized, leak-tight monolayers with measurable transepithelial electrical resistance (TEER) and expression of transport proteins typical of these regions. This approach provides a more physiologically relevant alternative to immortalized cell lines for investigating renal transport and barrier function (5).
RPEs: These cells form the outer blood-retina barrier, and when cultured on Transwell inserts, they allow researchers to study molecular exchange between the retina and choroid, which is the pigmented vascular layer of the eyeball. These models are used to evaluate retinal drug permeability, oxidative stress responses, and mechanisms of degenerative retinal diseases. A recent study showed that when differentiated from human induced pluripotent stem cells, RPE monolayers grown on Transwells can develop apical-basal polarity and functional tight junctions, enabling reproducible barrier formation and functional assessments such as TEER or permeability measurements (6).
Liver Sinusoidal Endothelial Cells (LSECs): LSECs form the fenestrated endothelial layer of the hepatic sinusoids, establishing the sinusoidal interface that governs molecular exchange between circulating blood and hepatocytes. When cultured on porous Transwell membranes, LSECs (or LSEC-like cell lines) develop selective permeability that can be assessed using small-molecule and macromolecular tracers such as Lucifer Yellow and FITC-dextrans.
Recently reported Transwell-based co-culture models with LSECs and hepatocytes (e.g., HepG2/C3a spheroids) show that this setup can mimic the sinusoidal interface. These models enable directional transport studies and reveal how drugs such as acetaminophen traverse and affect both endothelial and hepatic compartments. Although TEER values in LSEC monolayers tend to be low due to their fenestrated morphology, combining TEER or permeability measurements with junctional marker analysis, e.g., PECAM-1, stabilin-2, offers a reliable assessment of barrier integrity and function (7).
Are you interested in setting up or optimizing these models in your own lab?
Contact our support team (click here) to get our Transwell Assay Guides for practical tips, detailed protocols, and examples of TEER applications across different barrier-forming cell types.
References:
- Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015 20(2):107–126.
- Palma-Florez S., López-Canosa A., Moralez-Zavala F., et al. BBB-on-a-chip with integrated micro-TEER for permeability evaluation of multifunctionalized gold nanorods against Alzheimer’s disease. J Nanobiotechnol. 2023 21:115.
- OECD. Test No. 439: In vitro skin irritation: Reconstructed human epidermis test method. Paris: OECD Publishing; 2021. Accessed October 20, 2025
- OECD. Test No. 431: In vitro skin corrosion: Reconstructed human epidermis (RHE) test method. Paris: OECD Publishing; 2021.
- Dilmen E, Olde Hanhof CJA, Yousef Yengej FA, et al. A semi-permeable insert culture model for the distal part of the nephron with human and mouse tubuloid epithelial cells. Experimental Cell Research. 2025 444:114342.
- Ye, K., Takemoto, Y., Ito, A. et al. Reproducible production and image-based quality evaluation of retinal pigment epithelium sheets from human induced pluripotent stem cells. Sci Rep. 2020 Aug 25;10(1):14387.
- Poussin, C., Dumas, M., Attalla, R., et al. Co-culture model of a liver sinusoidal endothelial cell barrier and HepG2/C3a spheroids-on-chip in an advanced fluidic platform. J Biosci Bioeng. 2023 136(5):509–520.
Karen O’Hanlon Cohrt is an independent Science Writer with a PhD in biotechnology from Maynooth University, Ireland (2011). After her PhD, Karen relocated to Denmark where she held postdoctoral positions in mycology and later in human cell cycle regulation, before moving to the world of drug discovery. Karen has been a full-time science writer since 2017, and has since then held numerous contract roles in science communication and editing spanning diverse topics including diagnostics, molecular biology, and gene therapy. Her broad research background provides the technical know-how to support scientists in diverse areas, and this in combination with her passion for learning helps her to keep abreast of exciting research developments as they unfold. Karen is currently based in Ireland, and you can follow her on Linkedin here.
